Addition of Hydrogen Halides to Alkenes Mechanism
Organic Chemistry Study Materials Practice Problems Summary Sheet Guides Multiple-Choice Quizzes. However often the two are used interchangeably because the.
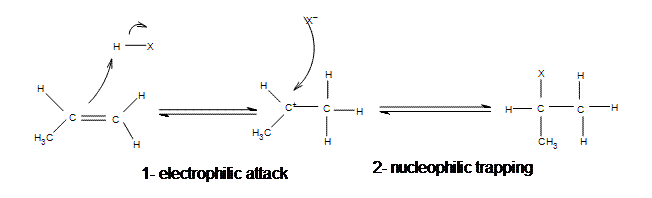
Electrophilic Addition Of Hydrogen Halides Chemistry Libretexts
CHCl COEt ACN CoEt AcOEt CN -78C 8h 25 75 When cyclopentadiene is added in excess the reaction is pseudo-1 order with respect to the fumaric nitrile ester.
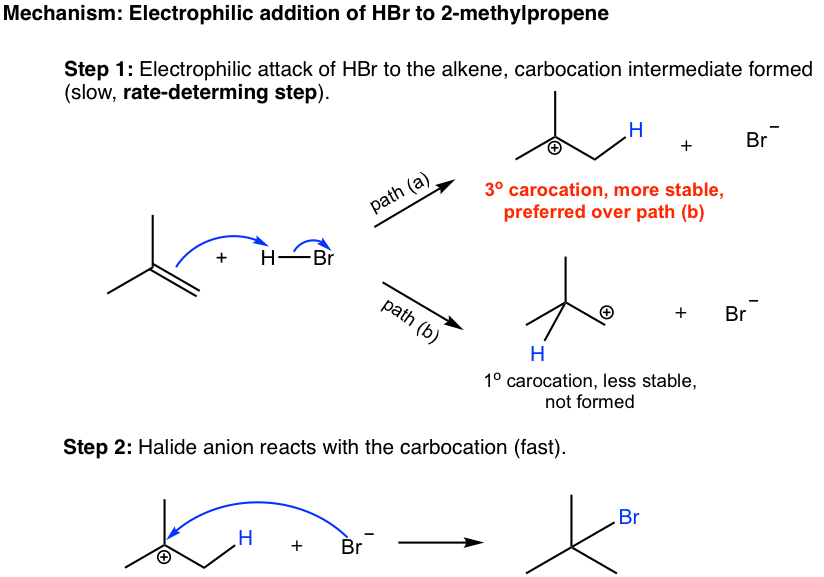
. Matching alcohols to their names I. The steps that are involved. Drawing formulas from names.
The mechanism of alkyne hydrogenation is identical to that of the alkenes. 1-butene 2-butene and isobutylene. The following Diels-Alder cycloaddition was recently performed by Mukherjee et al.
In case of unsymmetrical alkenes the addition reaction takes place in accordance with Markovnikovs rule Unit 13 Class XI. Similarly groups that favor ionization of the halogen may generate a transition state with substantial positive charge on the alpha-carbon and only a small degree of CH breaking. The elements of water can be added to the doublebonded carbons of an alkene in either a Markovnikovs or an antiMarkovnikovs manner.
Gabriel Phthalimide Synthesis was discovered by a German chemist named Siegmund Gabriel. Basically it follows a 3-step mechanism. The antiMarkovnikovs addition results from a hydroborationoxidation reaction.
This concept of redox active ligands has resulted in new base metal catalysts for the asymmetric hydrogenation of alkenes as well as the hydrosilylation and hydroboration of olefins. The ability of hydrocarbons to bond to themselves is known as catenation. The primary alcohols elimination reactions follow the E2 mechanism whereas the secondary and tertiary alcohols elimination reaction follows the E1 mechanism.
Drawing alkyne formulas from names. You will find it - Its all here. A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine.
The bond angles are approximately 120 as expected of a trigonal coplanar structure Figure 121. Mechanism The mechanism of the reaction involves the following three steps. Mechanism of Dehydration of Alcohols.
1212 Structure of the Carbonyl. Both steps in the above addition follow the Markovnikov rule. As shown in the following figure a hydrogen ion catalyzes the Markovnikovs addition.
Because the hydrogen is absorbed on the catalyst surface. Drawing alkene formulas from names. Theres no warning sign saying wait.
Drawing alcohol formulas. Visit Friedel Crafts Reaction for an in-depth explanation of the reaction details and mechanism. A recent application is the generation of highly reactive aryl radicals which are useful arylating reagents in synthesis by photoinduced electron transfer PET from photoredox catalysts to suitable precursors followed by bond scission 8 9However the choice of aryl radical precursors is currently limited to electron-poor arenes such as diazonium 6 10 or.
Thus the addition of hydrogen bromide to 1butyne gives 2bromo1butene as the major product of the. Drawing formulas from names. If its not in the textbook chances are it.
C 3 H 6. Drawing formulas from names. For instance alkanes alkynes or alkenes the amount of bonded hydrogen decreases in alkenes and alkynes.
From alkenes i By acid catalysed hydration. C 4 H 8. C 2 H 4.
Alkenes react with water in the presence of acid as catalyst to form alcohols. Our catalyst development efforts are often paired with industrial partners as our. With such capabilities they can.
In four textbooks where SOCl 2 is mentioned the reaction is shown as proceeding through an S N 2 mechanism. The Gabriel synthesis is a chemical reaction used to obtain primary amines from primary alkyl halides. For example if the Rgroups on the beta-carbon enhance the acidity of that hydrogen then substantial breaking of CH may occur before the other bonds begin to be affected.
Alkenes having four or more carbon atoms can form diverse structural isomersMost alkenes are also isomers of cycloalkanesAcyclic alkene structural isomers with only one double bond follow. Formation of alkenes. Dehydration of alcohols follows the E1 or E2 mechanism.
Given that i Ni0 complexes undergo oxidative addition more readily than NiI complexes with aryl halides and ii NiII complexes are believed to rapidly engage with sp 3 carbon-centered radicals to form NiIII species enabling sp 3 sp 2 and sp 3 sp 3 CC bond formations 15 16 we favor the dual-catalysis mechanism outlined in Fig. This is mainly due to the self-bonding or catenation of carbon that prevents the complete saturation of the hydrocarbon by the formation of double or triple bonds. The S N 2 doesnt happen for secondary alcohols.
In addition the oxygen atom also has two non bonding electron pairs. C 5 H 10. It is a subset of reactions that very closely resembles the insertion reactions and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products.
Thus the carbonyl carbon and the three atoms attached to it lie in the same plane and the π-electron cloud is above and below this plane. Hydrogen halides react with alkynes in the same manner as they do with alkenes. In each case the base metal catalysts offer unique function over established precious metals catalysts.
Only one textbook in this admittedly incomplete sample mentions the S N i mechanism at all.
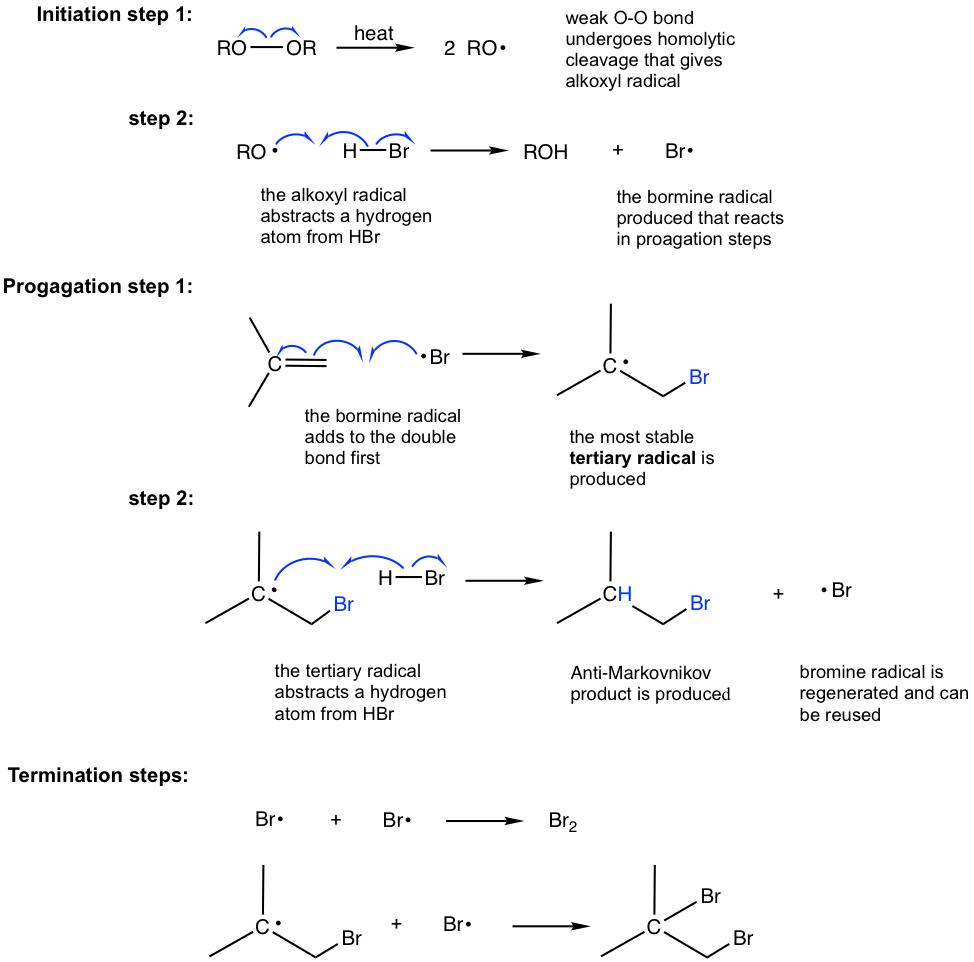
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I

10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I
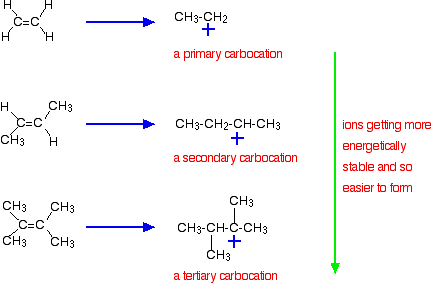
9 2 Addition Of Hydrogen Halides To Symmetrical Alkenes Chemistry Libretexts

Regioselectivity Of Hydrogen Halide Addition Markovnikov S Rule Youtube

Electrophilic Addition Of Hydrogen Halides To Alkenes Youtube

Electrophilic Addition Reactions Of Alkenes Mcc Organic Chemistry
0 Response to "Addition of Hydrogen Halides to Alkenes Mechanism"
Post a Comment